Introduction of Department
Hideki Kawanishi
Tsuchiya General Hospital, Hiroshima JAPAN
Encapsulating peritoneal sclerosis (EPS) is a serious complication of long-term peritoneal dialysis (PD). The mortality rate for EPS has been high, primarily because of complications related to bowel obstruction. However, recent advances in clinical research have established the pathogenesis and course and a treatment strategy. To date, there is no consensus on therapy, but medication care a trial of corticosteroids should be given, but a tamoxifen still remain controversial. The final therapeutic option for EPS is surgical enterolysis (adhesiolysis), and we have performed 239 surgical procedures in 181 patients and observed favorable outcomes.
In the 1990s, Japan experienced a large number of EPS cases, and PD therapy faced a crisis. There were many negative viewpoints on treatment of EPS at the beginning, but surgery became accepted in the face of an increasing number of cases, and several other facilities introduced surgical therapy. This activity promoted the understanding of, and countermeasures against, EPS in Japan, and EPS is no longer recognized as a fatal complication.
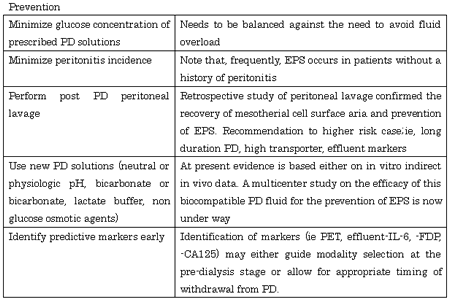
In encapsulating peritoneal sclerosis (EPS), intraperitoneal inflammation leads to adhesive and inflammatory encapsulation of the intestinal tract, which then manifests as bowel obstruction syndrome. With the widespread use of peritoneal dialysis (PD), the number of patients developing EPS, a potentially fatal PD-related complication, has increased (1,2). As a consequence, there is much debate about whether there should be an arbitrary expiry date for PD because of the risk of EPS. However, recent clinical studies have clarified the pathogenesis of EPS and have proposed therapeutic strategies (Table 1) (3).
In particular, the surgical option was previously contraindicated in patients with EPS (1); however, the final option for patients in whom bowel obstruction symptoms fail to improve is surgical enterolysis, and we have actively performed this procedure since 1993. In EPS, the intestine is degenerated and vulnerable, and so the risk of intestinal perforation is high because of persistent obstruction. Such occurrences are fatal. We therefore consider that surgery is indicated for all EPS patients with severe symptoms of bowel obstruction (4-8).
Currently, understanding for the EPS are, 1) EPS occurs in longer duration of PD, indicating the involvement of peritoneal deterioration; 2) EPS involves some kind of infection; 3) EPS frequently occurs after PD withdrawal and catheter removal; 4) Timely administration of corticosteroids is effective. 5) Surgical enterolysis (adhesiolysis) is the optimal treatment to relieve bowel obstructions.
EPS develops when PD therapy (mainly with bioincompatible dialysis solutions) causes peritoneal deterioration, and a capsule formed by accumulated fibrin covers the deteriorated intestine and becomes firm, thereby impairing intestinal peristalsis, leading to the appearance of bowel obstruction symptoms (9). The desquamation and disappearance of peritoneal mesothelial cells because of long-term exposure to PD solutions result in the progression of peritoneal fibrosis.
In addition, peritoneal capillary angiogenesis and hyperplasia develop, increasing peritoneal permeability (10). The new vascular vessels appear to exhibit abnormally increased endothelial permeability to high molecular-weight substances such as fibrin (9). In this manner, a fibrin membrane is formed on the surface of a thickened, fibrotic peritoneum.
Fibrin deposited during PD is washed away with the dialysis solution, resulting in mild capsule formation. However, after PD withdrawal, fibrin remains in the abdominal cavity and accelerates capsule formation. In addition, a complicating inflammation, particularly bacterial peritonitis, further increases peritoneal permeability, and causes the deposition of large amounts of fibrin, leading to rapid capsule formation and EPS development. However, mild functional deterioration of the peritoneum does not lead to the development of EPS, because even when inflammation occurs and large amounts of fibrin deposit, no capsule is formed on the peritoneal surface. In contrast, in cases of severe peritoneal deterioration, even a slight inflammation easily leads to the formation of capsules, with the resulting development of EPS. Thus, EPS develops depending on the balance between the severity of peritoneal deterioration and that of inflammation (11).
Development of EPS is based on deterioration of the peritoneum, and its rate of development is proportional to the duration of PD. Our prospective study showed that discontinuation of PD within 8 years reduces the risk of EPS development (2). However, it is dangerous to prescribe the duration (in years) of PD, and it is necessary to evaluate the degree of peritoneal deterioration.
The simplest method of evaluation is the peritoneal equilibration test (PET) (12,13). If the patient is classified as a high transporter, PD should be discontinued. In addition, prevention of peritoneal deterioration is important to prevent the development of EPS. For this purpose, it is important to use biocompatible dialysis solutions to reduce the glucose load. Recently, a new neutral pH dialysis solution in a two-compartment bag has become available (14). This solution contains smaller amounts of cytotoxic glucose degradation products and promises to prevent peritoneal deterioration.
Peritoneal Lavage: To remove fibrin, peritoneal lavage has been performed with a catheter left in place after withdrawal of PD. Retrospective study of peritoneal lavage confirmed the recovery of mesotherial cell surface aria and prevention of EPS (15). However, peritoneal lavage might not be expected to improve the deterioration of the peritoneum, only to prolong the time to thickening of the capsules, leading to the development of EPS. Peritonitis as a complication during peritoneal lavage has a reverse effect, inevitably leading to EPS.
In addition, it is problematic to physically stress a patient by leaving the catheter in place even after PD withdrawal, making it necessary to establish criteria to determine when to remove the catheter. In particular, there has been a recent trend toward performing peritoneal lavage in patients with a low risk of EPS, requiring strict indications for the procedure.
The indications for peritoneal lavage include, 1) long-term PD duration (more than 8 years); 2) peritoneal permeability increase diagnosed by PET; 3) increased levels of markers of inflammation, coagulation, fibrinolysis (IL-6, FDPs) in effluent, increased fibrin and/or protein in the effluent, a bloody effluent, and increase of mesothelial cells surface aria. A PET is performed every 3 months during peritoneal lavage, and dialysate-to-plasma ratios of (D/P) creatinine, dialysate levels of cancer antigen 125 and mesothelial cells surface aria are determined. If those values improve, the catheter should be removed (15,16).
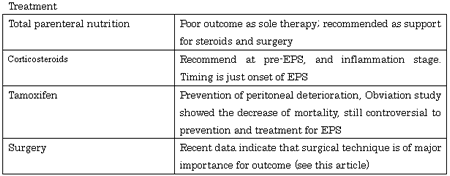
Although steroids are currently the first line drugs for the treatment of EPS, a prospective study showed that only 15 (38.5%) of 39 patients given steroids after EPS development achieved symptomatic improvement (2). Steroids have the effect of suppressing inflammation to prevent ascites and fibrin deposition, but to be effective, they must be used immediately after EPS development (17, 18). They are also effective in the pre-EPS stage when ascitic fluid increases. The timely administration of steroids terminates the inflammation and reduces ascitic fluid, thereby preventing progression to a state of bowel obstruction. However, delayed administration of steroids results in failure to prevent capsule formation, leading to the appearance of bowel obstruction symptoms. In rare cases, the administration of steroids is effective, but dose reduction results in recurrence in some patients. Their treatment poses a challenge, requiring careful dose reduction. If an effect is achieved, the dose should be continued for a long period.
Steroids cannot be expected to be effective for EPS associated with established bowel obstruction symptoms. If the CRP level is persistently elevated, steroid administration is continued, but gradually tapered, and surgical treatment is considered, as will be described shortly.
Is prophylactic steroid administration effective? The indications include 1) inflammatory cell infiltration confirmed by peritoneal biopsy before EPS development; 2) persistent CRP positivity in the absence of other infections; 3) a rapid increase in ascitic fluid; and 4) an increase in markers of inflammation, coagulation, and fibrinolysis (such as IL-6 and FDPs) in the effluent. However, it has not been demonstrated that prophylactic steroid administration prevents the development of EPS. In any event, steroids should been administered following strict indications.
Tamoxifen is a non-steroidal anti-estrogen used to treat carcinoma of breast. It is also used in treating fibrosing diseases such as fibrosing medianitis and retroperitoneal fibrosis. The administration of tamoxifen has been attempted mainly in Europe for the prevention of peritoneal fibrosis. However, its effectiveness remains unclear because of the limited number of patients (19-21). The proposed mechanism of action of tamoxifen is that it upregulates transforming growth factor β1 (TGF- β1), which stimulates metalloproteinase-9 to remove degenerated collagen, thereby preventing damage to mesothelial cells (19). Recent study of immunophenotyping of peritoneal biopsy specimens includes EPS cases showed the sparse expression of estrogen receptor of the peritoneal (22). Thus, the blockade of the estrogen receptor of tamoxifen is probably not the mechanism of action.
The multicenter Dutch study, EPS was retrospectively analyzed in 63 patients, and the efficacy of survival rate of tamoxifen was presented, and the mortality rate of tamoxifen group (n:24) was 45.8% compared non-tamoxifen group (n: 39) 63.5% (p<0.077), (23). However, other large retrospective Pan-Thames study could not define the effective of medical treatments included tamoxifen (24). Tamoxifen is administered at daily doses of 10-20 mg, and care should be taken in increasing the dose, because of the frequent development of complicating thrombotic lesions (21). From these findings, tamoxifen is still controversial to prevention and treatment for EPS. Larger prospective studies are necessary to confirm of effects of these medications.
Our first encounter with a patient with EPS who underwent surgical enterolysis (adhesiolysis) occurred in 1993; that patient was completely cured. From then until the end of 2010, we performed 239 enterolysis procedures in 181 patients (8). Of those 181 patients, 14 died after surgery; all of the others showed improvement.
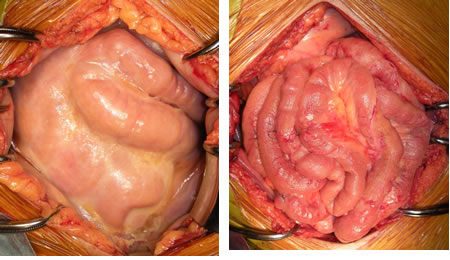
Considering the mechanism of EPS development, the surgical technique is simple, involving only the division of peritoneal adhesions by repeated lysis of fibrin membranes with a sharp instrument (Figure 1). Recently, to identify the site of stenosis, we have, after enterolysis, been inserting a Miller-Abbott ileus tube with an inflated balloon to the end of the ileum.
Surgery can reverse the bowel obstruction, but it does not improve the peritoneal deterioration. As a result, the capsules can re-form, and EPS can recur in some patients 6-12 months later. In addition, adhesions also occur as a result of surgical injury to the intestinal wall and mesenteric serosa. To prevent recurrences, we have, since April 2007, been performing the Noble plication procedure (25,26), in which intestine is sutured to intestine to prevent re-obstruction of the bowel. This technique prevents not only passage disturbances resulting from kinking and adhesion of the small intestine, but also escape into and adhesions in the pelvic cavity. In patients experiencing recurrence or presenting difficulties in complete adhesiolysis because of intestinal wall calcification require bypass between the oral site jejunum and the ileum or large intestine.
Most of the 14 patients (7.7%) who died postoperatively, died of sepsis resulting from intestinal perforation and infection; 1 died from hepatic failure. Enterolysis was performed in 169 first surgeries; the Noble plication was added in 57 recent cases. Bypass between the oral site jejunum and the ileum or large intestine was performed in 9 patients in whom enterolysis could not be performed. In 3 patients with localized adhesions and mild degeneration of the wall of the small intestine, the adhered small intestine was resected and anastomosed.
Of 112 patients treated solely with enterolysis in the first surgery, 34 (30.4%) required re-surgery. In 57 patients, enterolysis with Noble plication was performed in the first surgery; 7 of those patients (12.3%) required re-surgery. We compared the course of re-surgery between patients who underwent the Noble plication procedure and those who underwent enterolysis alone for their initial surgery. Although a long-term comparison is difficult because the follow-up period for the Noble plication group is short, the 1- and 2-year rates of freedom from a re-surgery are higher in the group treated with Noble plication (0.91 vs. 0.76 and 0.81 vs. 0.70 respectively), suggesting that the Noble plication is effective in preventing recurrence (8).
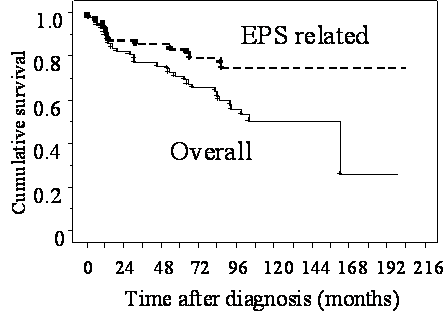
At the end of 2010, outcomes in 6 of the 181 patients were unknown. Excluding those 6 patients, the mean duration of postoperative follow-up was 46.4 months (range: 0.3-208 months). A total of 64 patients who opted for surgery (35.4%) died. Death was related to EPS in 33 patients (18.2%), including the 14 who died postoperatively (Table 2). The overall survival rate at 1, 2, 3, 5, and 8 years after diagnosis was 93%, 83%, 78%, 71%, and 60% respectively. The survival rate for non-EPS-related death at 1, 2, 3, 5, and 8 years after diagnosis was 95%, 90%, 87%, 81%, and 74% respectively. Median survival after diagnosis, considering death from any cause and death from EPS, was 43.9 months and 35.7 months respectively (Figure 2) (8).
Previously, the literature contained only case reports of the use of the surgical option for EPS (27-29). Surgery was previously contraindicated in patients with EPS, and most patients treated surgically died of peritonitis as a postoperative complication (1). These deaths occurred because the pathogenesis of EPS was not well understood by surgeons, and in many cases, simple resection of adherent intestinal loops with enteroanastomosis was performed by surgeons who had never been involved with PD.
We developed a surgical technique of total intestinal enterolysis without enterectomy, and since then, we have treated patients in the belief that surgical therapy is the only curative treatment for established EPS (4-8). In the period between 1993 and the end of 2010, we performed 239 enterolysis procedures in 181 patients. Of those 181 patients, 14 died after surgery; all of the others showed improvement.
The mortality rate from EPS has been reported to be 24%-66%, but findings lack clarity because of variations in the follow-up periods and treatment methods. The results of a relatively long-term follow-up have recently been reported. In the Pan-Thames study, in which EPS was observed in 111 patients, the overall mortality and 1-year overall survival rates were 53% and 56% respectively (24). In the Australia and New Zealand Dialysis and Transplant Registry, the overall mortality rate in 33 EPS patients was 55%, and the 1-, 2-, 3-, and 5-year survival rates were 69%, 62%, 58%, and 35% respectively (30). In a multicenter Dutch study, EPS was retrospectively analyzed in 63 patients, and the efficacy of tamoxifen was presented, but the overall mortality rate was 63.5%, and the 1-, 2-, and 3-year survival rates in the tamoxifen group (24 patients with an overall mortality rate of 45.8%) were 80%, 75%, and 60% respectively (23). Compared with these recent reports, outcomes in our study were markedly favorable: the overall mortality rate was 35.4% and the 1-, 2-, 3-, and 5-year survival rates were 93%, 83%, 78%, and 71% respectively (Table 2).
Given that the observation period in our study was 17 years, the severity of EPS may have changed, and the surgical techniques have been modified. Moreover, the therapeutic results were collected at a single facility and so cannot be directly compared with results collected at multiple facilities. However, the usefulness of surgical therapy for EPS has not been ruled out because all surgeries were performed by the same operator and surgical team under a set therapeutic policy.
In the 1990s, Japan experienced a large number of EPS cases, and PD therapy faced a crisis (1). There were many negative viewpoints on surgical treatment of EPS at the beginning, but surgery became accepted in the face of an increasing number of cases, and several other facilities introduced surgical therapy. This activity promoted the understanding of, and countermeasures against, EPS in Japan, and EPS is no longer recognized as a fatal complication. To improve the surgical results, a surgical team with a thorough understanding of the pathology of EPS is essential, for which the establishment of a regional EPS treatment center in each community and the training of surgeons are necessary.
In addition, biocompatible PD fluid (fluid low in glucose degradation products) became available for all patients, which may have reduced the EPS risk. A multicenter study on the efficacy of this biocompatible PD fluid for the prevention of EPS is now under way (31).
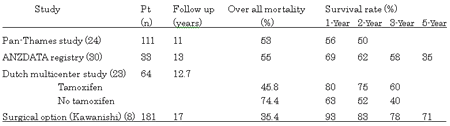
Left: at laparotomy, Right: after complete enterolysis